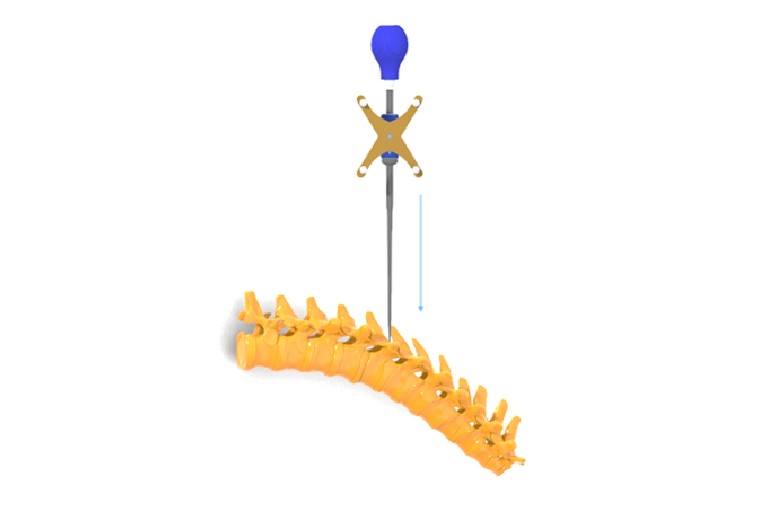
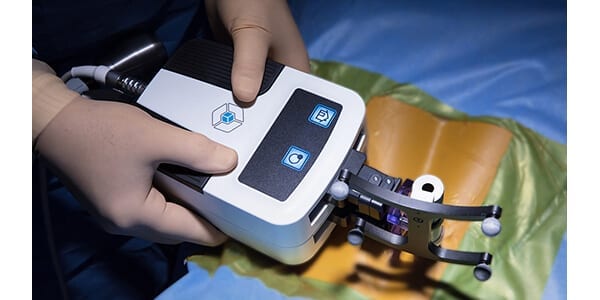
CTL Amedica granted FDA 510(k) approval for Navigation Instrument
4.5
(470)
Écrire un avis
Plus
€ 24.50
En Stock
Description

FDA grants 510k clearance of the CTL Amedica Navigation Instrument
_%20Substantial%20Equivalence%20Through%20Performance%20Criteria.png)
Abbreviated 510(k): Substantial Equivalence Through Performance

Anthony Strzalek, Author at Spinal News International

Connor Anderson - MetaGen Medical

NEWS Archives - Page 55 of 194 - SPINEMarketGroup

Suzie Marshall, Author at Spinal News International

Substantial Equivalence in a Predicate Device
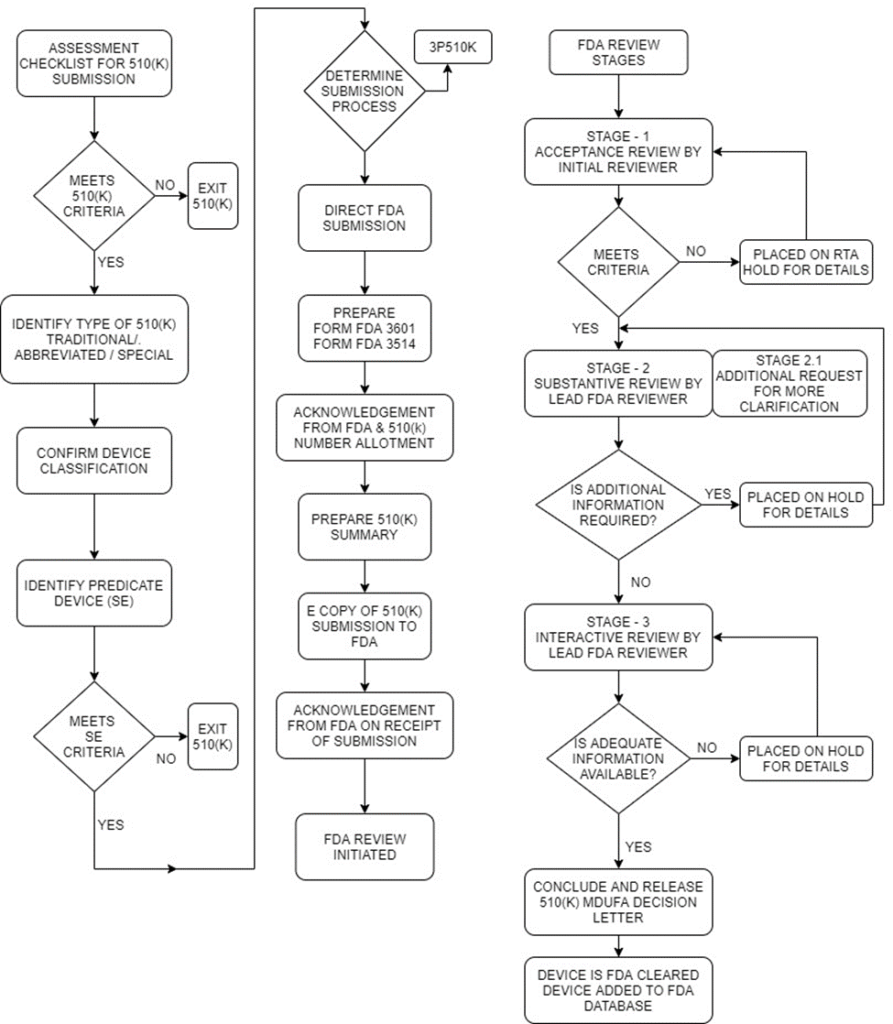
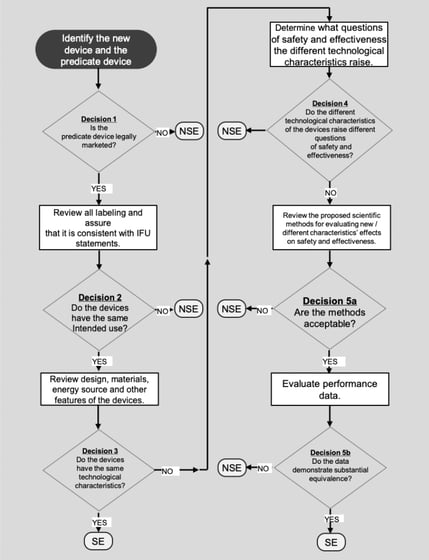
FDA's 510(K) Submission Process
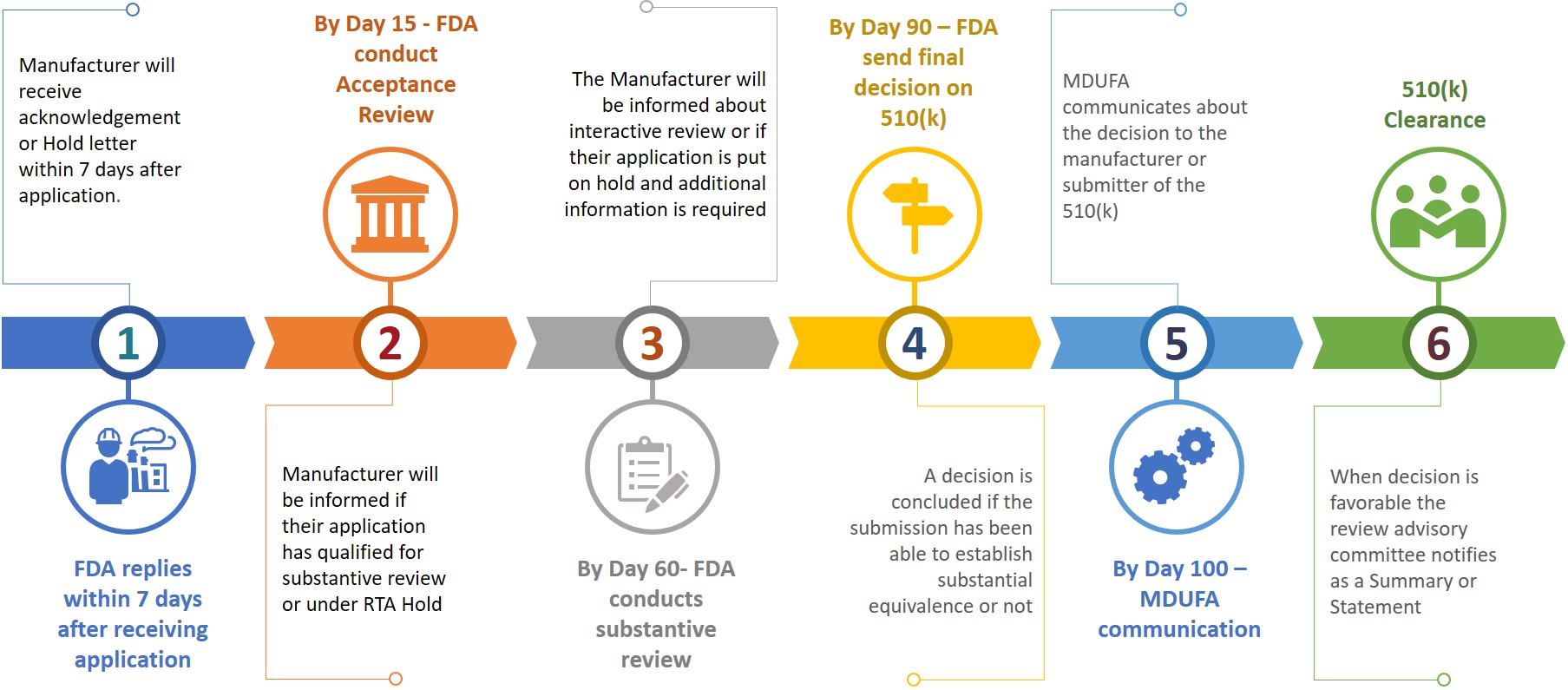
FDA 510k Premarket Notification: Essential Requirements

Frontiers The affinity of antigen-binding domain on the

Recent FDA 510(k) Clearances in Spine
Fusion Robotics Gains 510(k) for Spinal Navigation & Robotics
Everything you need to know about the FDA 510(k) submission

NEWS Archives - SPINEMarketGroup
Proposer des recherches
Tu pourrais aussi aimer