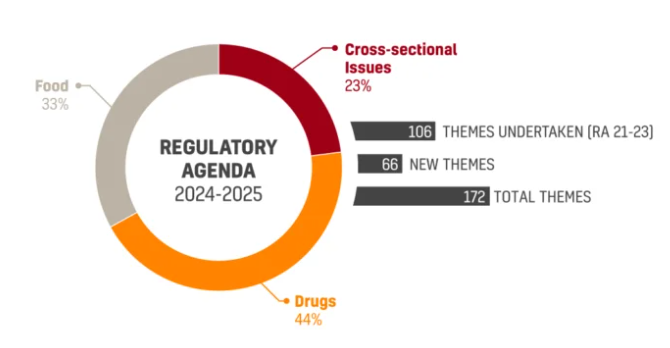
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

New FDA Approved Drugs & Devices to Watch for in 2024

Announcements ALTEX - Alternatives to animal experimentation

425

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

SEC Filing Alkermes plc

Orla Morrissey (@OrlaMorris61218) / X
FDA Grand Rounds - February 8, 2024 @ 12.pm. ET - US FDA

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

EU/USA - Regulation of Natural Food Additives — Food Compliance International

Inline XBRL Viewer

Sports betting is now regulated in Brazil

Inline XBRL Viewer

Brazil – ANVISA updates Regulatory Agenda for the year 2023