
Does The Difference In Structure Make Graphite Soft But Diamond Hard?

The Atomic Difference Between Diamonds and Graphite – Sustainable Nano

Shock-formed carbon materials with intergrown sp3- and sp2-bonded nanostructured units
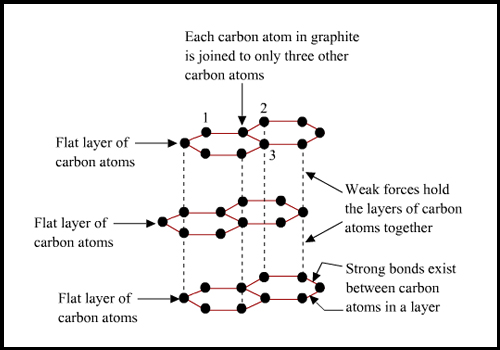
Describe why diamond is hard and graphite is soft?
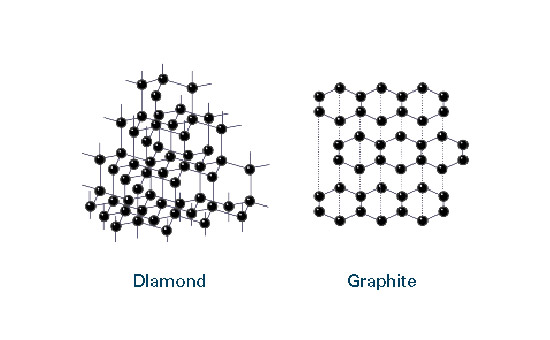
What is graphite? - ECGA
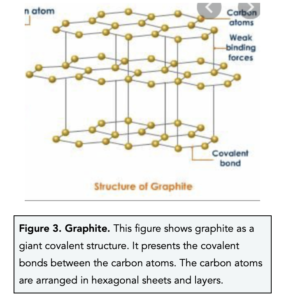
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) - Study Mind

Does The Difference In Structure Make Graphite Soft But Diamond Hard?

Why diamond and graphite have different physical properties but same chemical properties? What is the property called? - Quora
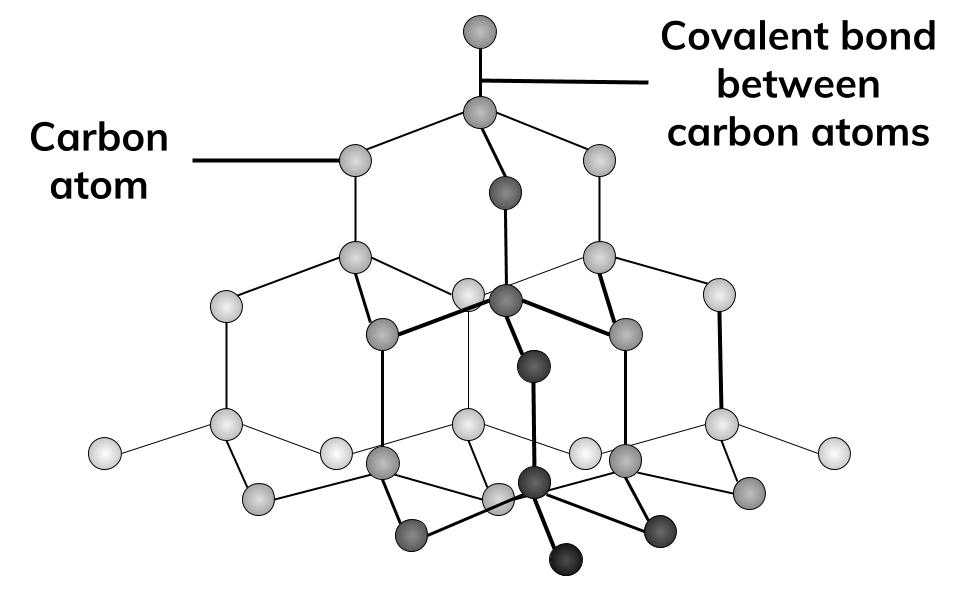
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

Carbon : The Sparkling Simplicity of Nature's Diamonds

Describe why diamond is hard and graphite is soft?
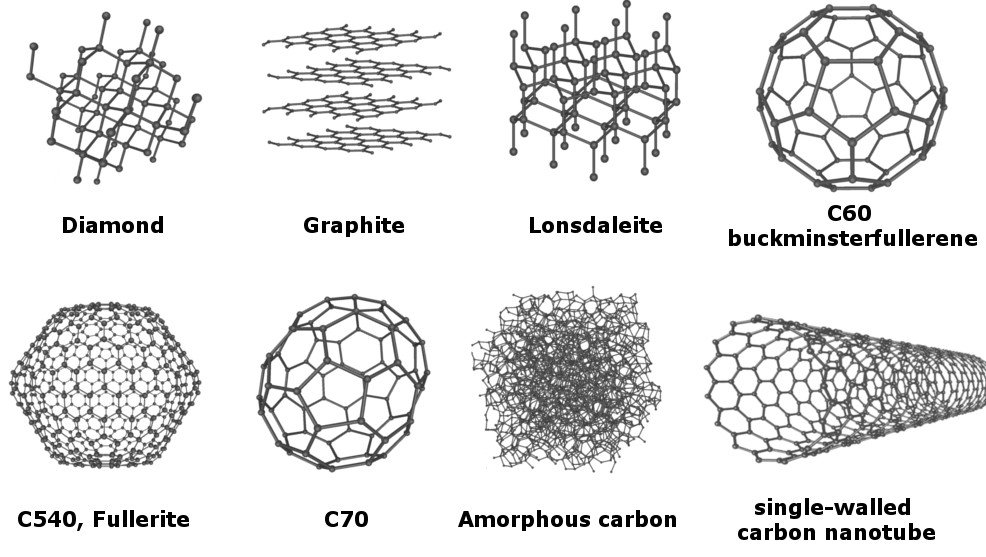
Does The Difference In Structure Make Graphite Soft But Diamond Hard?
.jpg)








