Risk of Guillain–Barré syndrome after meningococcal conjugate vaccination - Velentgas - 2012 - Pharmacoepidemiology and Drug Safety - Wiley Online Library

GBS Vaccine Injury Law Firm - Schiffmacher Cinelli Adoff LLP
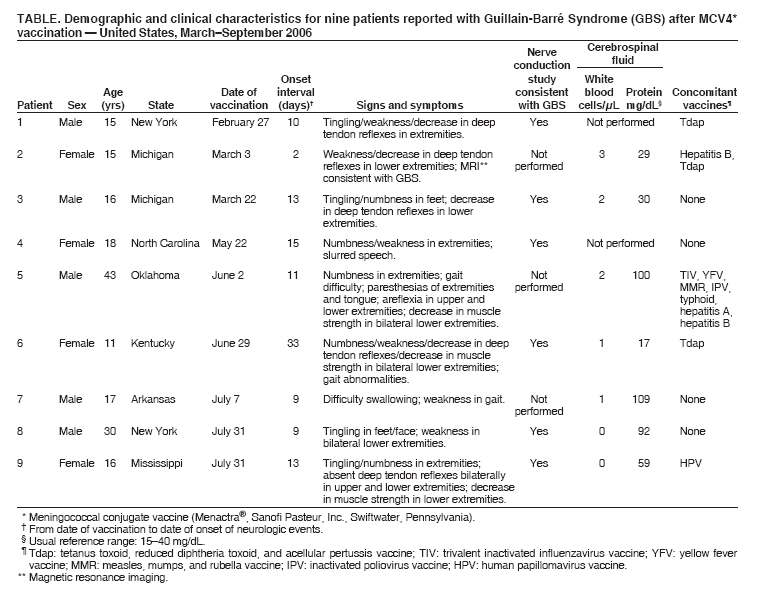
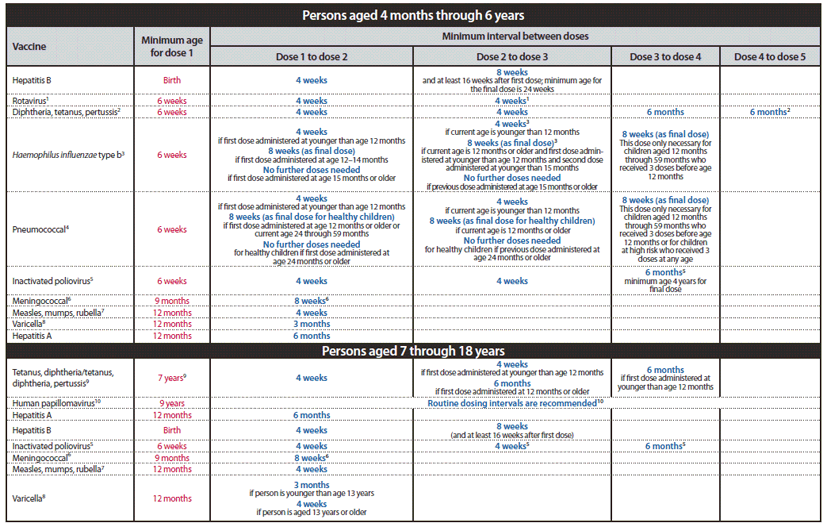
Update: Guillain-Barré Syndrome Among Recipients of Menactra® Meningococcal Conjugate Vaccine --- United States, June 2005--September 2006

Pharmacoepidemiology [6th Edition] 9781119413370
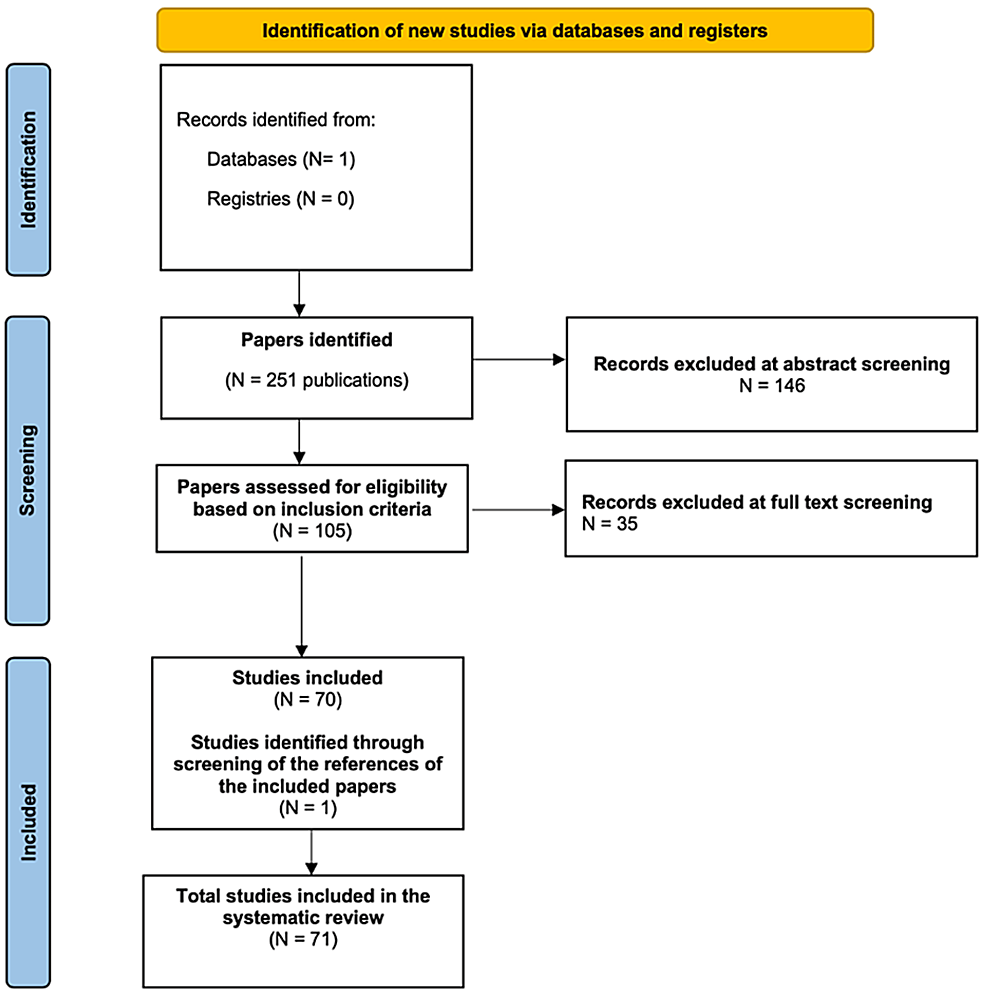
Cureus, Guillain-Barré Syndrome Induced by Vaccination Against COVID-19: A Systematic Review and Meta-Analysis

Guillain–Barré syndrome and COVID-19 vaccination: a systematic review and meta-analysis

Risk of Acute Liver Injury Associated with the Use of Moxifloxacin and Other Oral Antimicrobials: A Retrospective, Population‐Based Cohort Study - Kaye - 2014 - Pharmacotherapy: The Journal of Human Pharmacology and

Risk factors for the severity of Guillain-Barré syndrome and predictors of short-term prognosis of severe Guillain-Barré syndrome

An International Perspective on Preceding Infections in Guillain-Barré Syndrome

Risk of Guillain-Barré syndrome after meningococcal conjugate vaccination