IQ, OQ, PQ: A Quick Guide to Process Validation
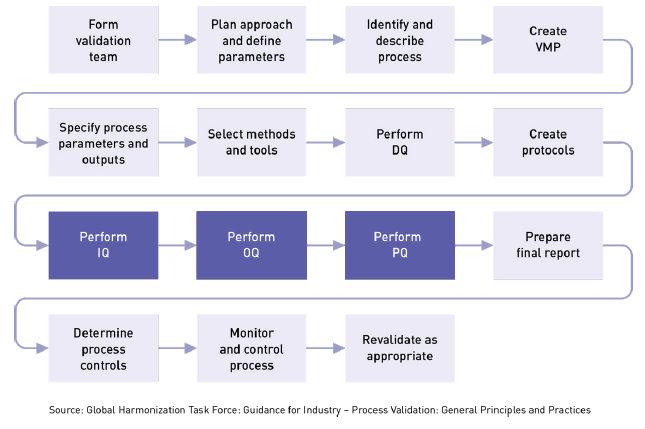
Learn about process validation and its three phases: installation qualification (IQ), operation qualification (OQ), and performance qualification (PQ).
Pharmaceutical qualification and validation: tips to get through nightmares

Validation Qualifications IQ OQ PQ : PresentationEZE

IQ, OQ, PQ - A Guide to Process Validation by IZiel Group - Issuu
Overview of Medical Device Process Validation: IQ, OQ, and PQ – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
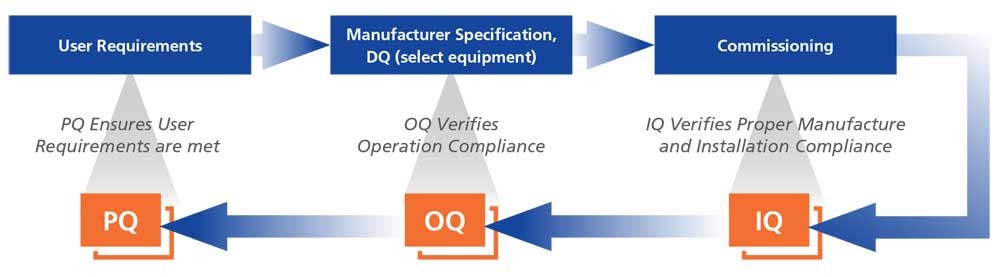
What Are DQ, IQ, OQ, and PQ, and Why Are They Required in Medical Device Industry?

IQ, OQ, PQ: A Quick Guide To Process Validation, PDF, Verification And Validation
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries

The 3 Q's in Computer System Validation - IQ OQ PQ - eLeaP
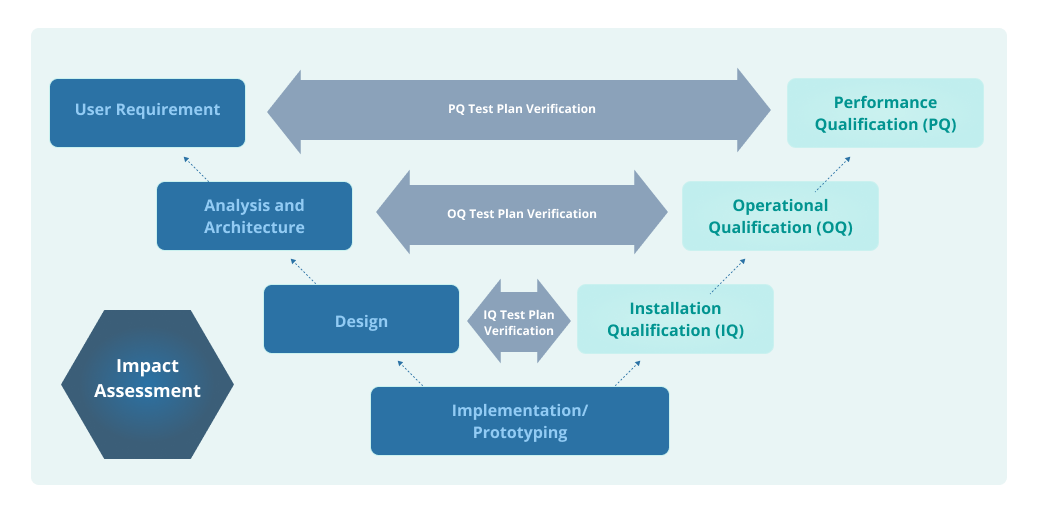
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries
Installation Qualification (IQ), Operational Qualification (OQ) & Performance Qualification (PQ) || Medical Devices

ISO 13485 - IQOQPQ - Process Validation for Medical Devices

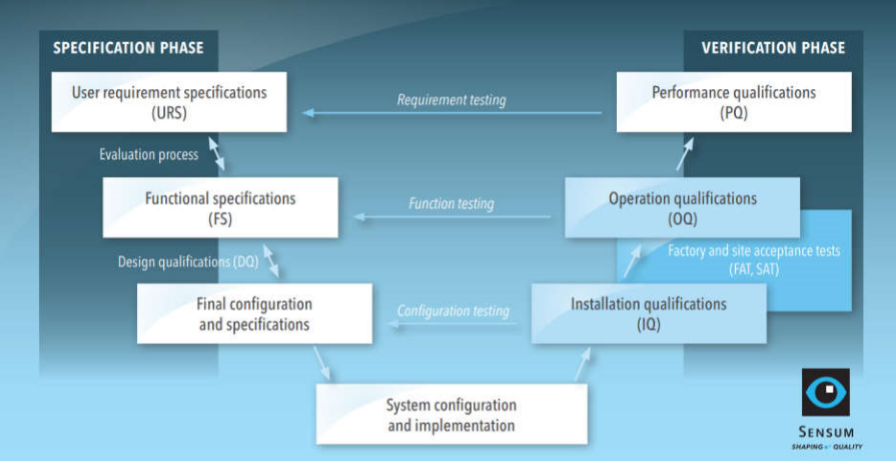
Concept of URS,DQ,IQ,OQ,PQ